

TITAN PHARMACEUTICALS CORPORATE PRESENTATION | SEPTEMBER 2018 © 2018 TITAN PHARMACEUTICALS INC. Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration No. 333 - 226841 September 4, 2018

This presentation contains forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, or the Securities Act, and Section 21 E of the Securities Exchange Act of 1934 , or the Exchange Act . All statements other than statements of historical facts contained or incorporated by reference in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward - looking statements . These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . These forward - looking statements are only predictions and we may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, so you should not place undue reliance on our forward - looking statements . Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward - looking statements we make . We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and operating results . We have included important factors in the cautionary statements included in this presentation, and in the documents incorporated by reference, particularly in the ‘Risk Factors’ section, that could cause actual future results or events to differ materially from the forward - looking statements that we make . Our forward - looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make . The forward - looking statements included in this presentation represent our views as of the date of this presentation . We anticipate that subsequent events and developments will cause our views to change . However, while we may elect to update these forward - looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law . You should, therefore, not rely on these forward - looking statements as representing our views as of any date subsequent to the date of this presentation . This presentation contains estimates made, and other statistical data published, by independent parties and by us relating to market size and growth and other data about our industry . We obtained the industry and market data in this presentation from our own research as well as from industry and general publications, surveys and studies conducted by third parties . This data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty . We caution you not to give undue weight to such projections, assumptions and estimates . ProNeura is a trademark and Probuphine is a registered trademark of Titan Pharmaceuticals, Inc . FORWARD - LOOKING STATEMENTS 2

This presentation highlights basic information about us and the offering to which this communication relates. Because it is a su mmary, it does not contain all of the information that you should consider before investing in our securities. We have filed a registration statement (including a prospectus, which currently is in preliminary form) with the U.S. Securit ies and Exchange Commission (“SEC”) for the offering to which this presentation relates. The registration has not yet become effective. Before you inves t, you should read the preliminary registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more co mplete information about us and this offering. You may access these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov. The preliminary prospectus, dated August 30, 2018, is available on the SEC Web site at www.sec.gov/Archives/edgar/data/910267/000157104918000460/tv501058 - s1a.htm . Alternatively, we or any underwriter participating in the offering will arrange to send you the preliminary prospectus and, w hen available, the final prospectus and/or any supplements thereto if you contact A.G.P./Alliance Global Partners, 590 Madison Avenue, 36th Floor, New Yo rk, NY 10022 or via telephone at 212 - 624 - 2006 or email: presentation@allianceg.com . This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of the se securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the se curities laws of such state or jurisdiction. The offering will only be made by means of a prospectus pursuant to a registration statement that is filed with the SEC after such registration statement becomes effective. 3 FREE WRITING PROSPECTUS STATEMENT

Exchange/Symbol NASDAQ:TTNP Offering Approximately $15 million Over - Allotment Option 15% Use of Proceeds Operations and other general corporate purposes, including building our infrastructure to commercialize Probuphine, conduct Phase IV trials required by the FDA and general working capital Underwriters Sole Book - Running Manager: A.G.P./Alliance Global Partners Co - Manager: Brookline Capital Markets (A Division of CIM Securities, LLC) OFFERING SUMMARY 4

TITAN LEADERSHIP RELEVANT EXPERIENCE Marc Rubin, M.D. Executive Chairman & Director • 12 years with Titan • Former Head of Global R&D and member of the Board of Management at Bayer Pharma • Executive R&D and commercial responsibilities at GSK for 13 years • 28 years in the pharmaceutical industry following 7 years at NIH Sunil Bhonsle President, CEO & Director • 22 years with Titan • Former Vice President and General Manager, Plasma Operations at Bayer Corporation • 20 years with Bayer in positions of increasing responsibility in the Biological and Pharmaceutical finance and operations management Kate Beebe DeVarney , Ph.D. Executive Vice President, Chief Scientific Officer • 12 years with Titan • 22 years in industry, with senior positions in clinical development and medical affairs at GSK, Merck and Corcept Therapeutics • 10 years in academic medicine - University of Pennsylvania, George Mason University, Yale University Federico Seghi Recli Lead Independent Director • 24 years within the pharmaceutical industry • Former CEO of Molteni & C. Dei Frattelli Alitti Società Di Esercizio S.P.A. ( Molteni ); led its successful transformation into a specialty pharmaceutical company focused on innovative products for the treatment of pain and addiction in Europe • President and CEO, Merck S.p.A, 2002 - 2005 5

COMPANY SNAPSHOT Transforming into a commercial - stage company following reacquisition of Probuphine ® rights in the U.S. Lead product, Probuphine, is an implant formulation of buprenorphine for the long - term maintenance treatment of opioid use disorder (OUD) • Approved in U.S. and Canada, under regulatory review in EU • An important therapeutic option to fight the growing opioid addiction pandemic ProNeura™ platform technology provides continuous delivery, maintaining a stable blood level of selected drugs for the treatment of addiction and other disease categories • Pain, Parkinson’s disease, malaria, diabetes, thyroid disease 6
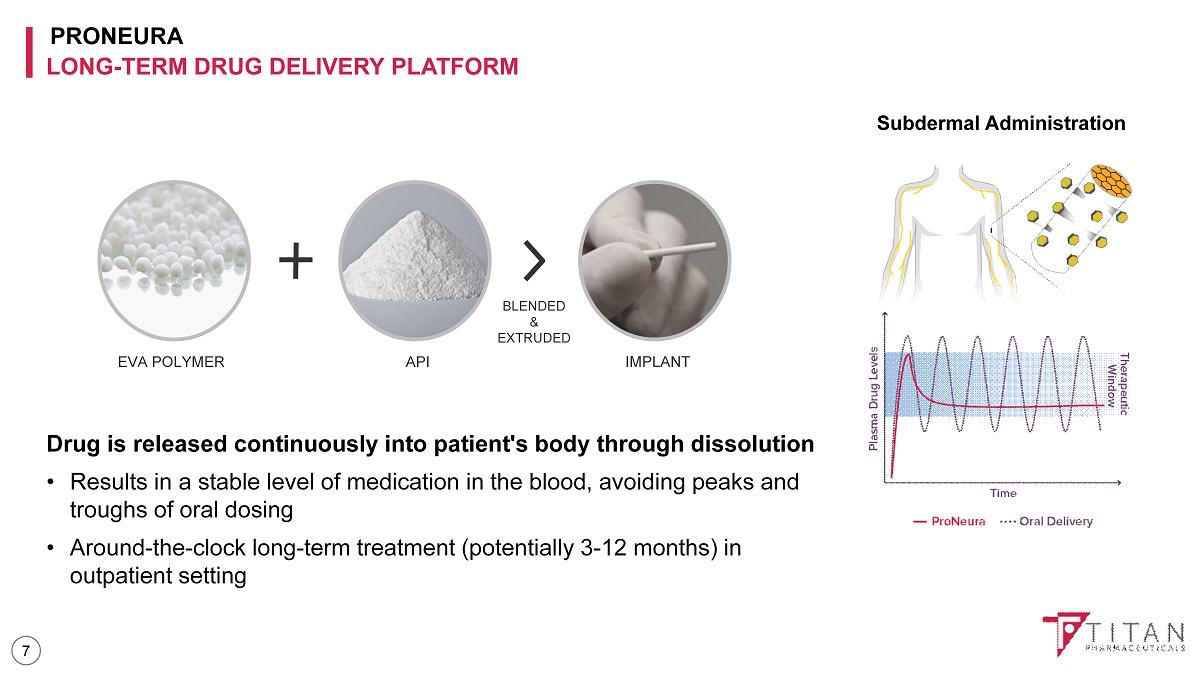
PRONEURA LONG - TERM DRUG DELIVERY PLATFORM Drug is released continuously into patient's body through dissolution • Results in a stable level of medication in the blood, avoiding peaks and troughs of oral dosing • Around - the - clock long - term treatment (potentially 3 - 12 months) in outpatient setting EVA POLYMER API IMPLANT BLENDED & EXTRUDED Subdermal Administration 7
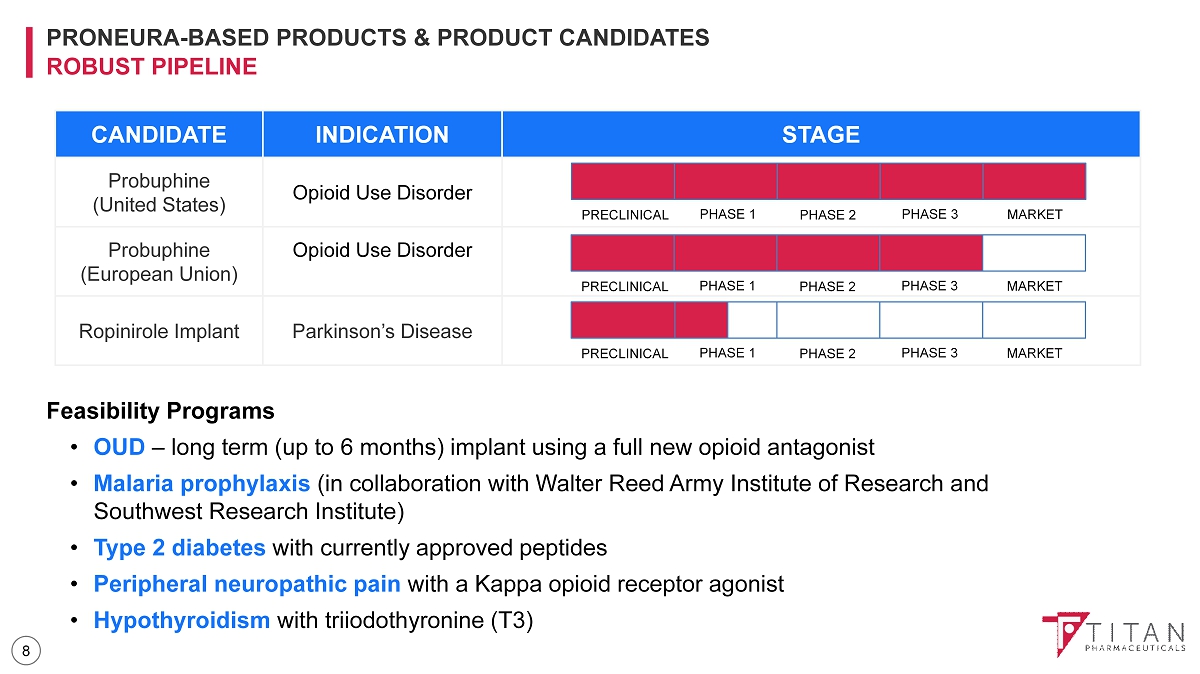
PRONEURA - BASED PRODUCTS & PRODUCT CANDIDATES ROBUST PIPELINE Feasibility Programs • OUD – long term (up to 6 months) implant using a full new opioid antagonist • Malaria prophylaxis (in collaboration with Walter Reed Army Institute of Research and Southwest Research Institute) • Type 2 diabetes with currently approved peptides • Peripheral neuropathic pain with a Kappa opioid receptor agonist • Hypothyroidism with triiodothyronine (T3) CANDIDATE INDICATION STAGE Probuphine (United States) Opioid Use Disorder Probuphine (European Union) Opioid Use Disorder Ropinirole Implant Parkinson’s Disease PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET 8

ADDICTION TO OPIOIDS A GLOBAL HEALTH EMERGENCY 9 A severe, chronic, relapsing brain disease characterized by compulsive drug seeking and use, despite harmful consequences • Cravings, accompanied by lack of impulse control • Cycles of relapse and remission • Progressive, and if untreated often leads to disability or premature death • Long - term treatment is necessary to reverse the effects of opioids on the brain Treatment approaches: • Medication Assisted Therapy (MAT) • Drug counseling • Abstinence - based programs (e.g. “12 step”)
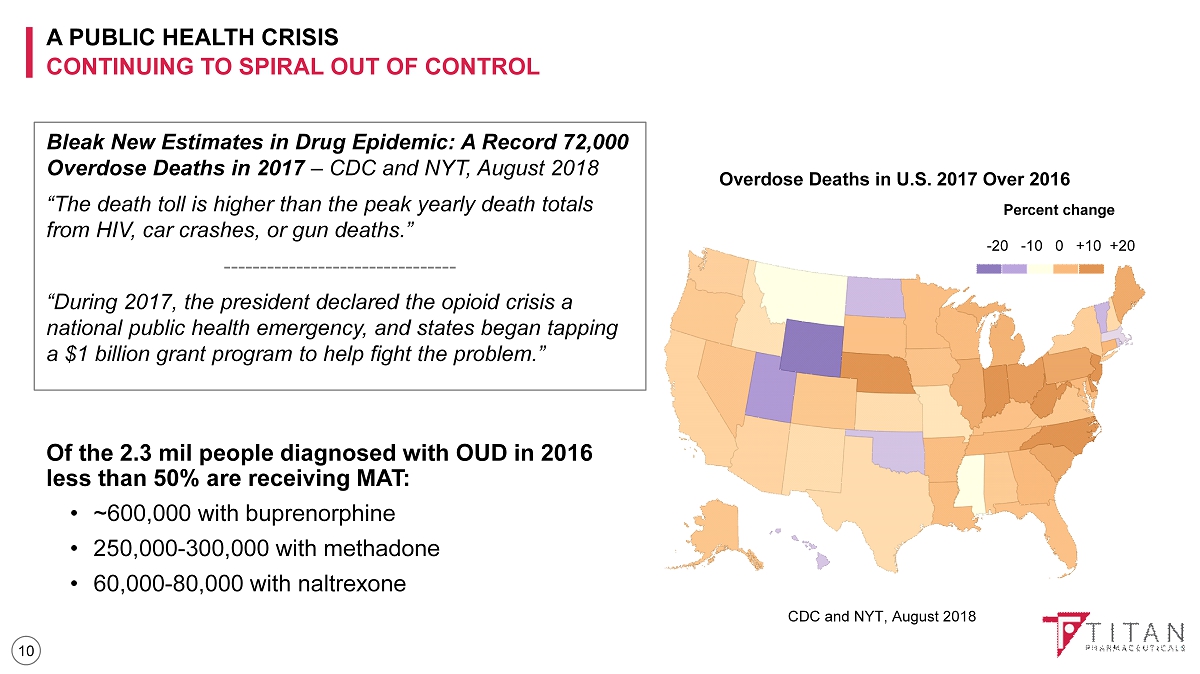
A PUBLIC HEALTH CRISIS CONTINUING TO SPIRAL OUT OF CONTROL 10 Bleak New Estimates in Drug Epidemic: A Record 72,000 Overdose Deaths in 2017 – CDC and NYT, August 2018 “The death toll is higher than the peak yearly death totals from HIV, car crashes, or gun deaths.” -------------------------------- “During 2017, the president declared the opioid crisis a national public health emergency, and states began tapping a $1 billion grant program to help fight the problem.” Percent change - 20 - 10 0 +10 +20 Overdose Deaths in U.S. 2017 Over 2016 Of the 2.3 mil people diagnosed with OUD in 2016 less than 50% are receiving MAT : • ~600,000 with buprenorphine • 250,000 - 300,000 with methadone • 60,000 - 80,000 with naltrexone CDC and NYT, August 2018

CURRENT MAT PARADIGM CHALLENGE & OPPORTUNITY 11 Buprenorphine is the gold standard • Approximately $2 billion annual sales of buprenorphine in U.S. Buprenorphine pharmacology: a safer, more effective, and more convenient treatment option • Controls withdrawal symptoms and cravings without inducing opioid euphoria • Low risk of respiratory depression Challenges with sublingual buprenorphine potentially addressed with Probuphine • Poor compliance • Diversion and abuse associated with current daily dosed formulations • Daily administration - potential reinforcement of drug - seeking behavior • Daily dosing results in variable levels of medication in blood • Stigma associated with daily dosing

PROBUPHINE A NOVEL SOLUTION 12 Regulatory Status: • U.S. Food and Drug Administration (FDA) approved in Q2 - 2016 - Transformational buprenorphine treatment for OUD • Health Canada approved in Q2 - 2018 - Sublicensed to Knight Therapeutics Inc. • Currently under review by the European Medicines Agency (EMA) *European patent was acquired by Molteni in March 2018 Intellectual Property: • Patent protection in U.S. and Europe* into 2024 and 2023, respectively • Related patents have also been issued in Australia, Canada, India, Japan, Mexico, New Zealand; application pending in Hong Kong

BRAEBURN U.S. PROBUPHINE LAUNCH MISSED OPPORTUNITY 13 Initial U.S. commercialization timeline: • FDA approved in May 2016 • Launched by Braeburn in July 2016 Braeburn’s launch missed the mark • Failure to appropriately segment the market at launch • Inadequate systems to support distribution, reimbursement and patient/physician education • Strategy limited to training large number of physicians (2,500+) • Essentially abandoned sales and marketing efforts in January 2018 Titan reacquired rights in May 2018

MAT ACCESS IS STARTING TO INCREASE 14 • In the U.S., 50% of the patient population with OUD is still medically untreated • About 52,000 physicians, representing 5 % of the nation’s doctors, are currently certified to prescribe buprenorphine • About 6,000 physicians writing approximately 90% of buprenorphine prescriptions • Still, about 1/2 of U.S. counties don’t have a single buprenorphine prescriber * • Excerpt from New York Times , Editorial Board, August 24, 2018 “In 2017, overdose deaths in the United States jumped 10% to about 72,000, the CDA said last week. The new data show that people are dying from opioids that are more potent and more dangerous than were available in years past. The CDC also found that many people who overdose are simultaneously using multiple drugs like heroin, fentanyl, cocaine, methamphetamines and benzodiazepine, an anti - anxiety medicine, and that the crisis has spread across the country, from rural and suburban areas to cities. Given all this grim news, the areas where overdose deaths are decreasing – Hawaii, Massachusetts, North Dakota, Oklahoma, Rhode Island, Utah, Vermont and Wyoming, per the CDC – stand out. Some of these states and cities have been at the forefront of increasing access to anti - overdose medicine naloxone and to anti - addiction medicines like buprenorphine and methadone, which experts say can help people who are dependent on opioids live relatively normal lives.” * New York Times, June 23, 2018
TREATMENT LANDSCAPE IS EVOLVING NEW TAILWINDS FOR THE RELAUNCH OF PROBUPHINE 15 The field is accelerating towards longer duration buprenorphine therapy • Initially led by Indivior PLC’s SUBLOCADE™ (buprenorphine extended - release) monthly depot injection, approved by FDA in November 2017 Indivior is devoting significant resources towards moving the market from daily to longer - term therapy • “Indivior remains confident in annual peak revenue goal of $ 1billion +”* We believe Probuphine will benefit • Only FDA - approved subdermal implant designed to deliver buprenorphine continuously for six months following a single treatment • No other products in development that allow for more than 30 days sustained therapy * Indivior Half Year Results 2018 presentation, July 25, 2018

Build a commercial infrastructure to support growth • Establish a small (~10 people) commercial and medical affairs team with expertise in: - Sales - Marketing and supply chain logistics - Medical science liaison and training functions - REMS program management - Third party payer and medical access Simplify system of distribution and reimbursement Focused market segmentation strategy Pursue additional partnerships for expanded market access TITAN IS TRANSFORMING DEVELOPMENT - STAGE TO COMMERCIAL - STAGE 16

RECENT MOLTENI PARTNERSHIP PROVIDES GLOBAL PHARMACEUTICAL INDUSTRY EXPERTISE 17 Molteni • Recognized leader in the field of drug dependence, operating in more than 30 countries • Strong track record of launching and commercializing innovative pain and addiction pharma products • Federico Seghi Recli (former CEO of Molteni ) appointed to Titan’s Board of Directors in May 2018 Acquired Probuphine European intellectual property • Includes the Marketing Authorization Application (MAA) currently under review by the EMA • Exclusive right to commercialize Titan - supplied Probuphine product in Europe (plus certain countries of the Commonwealth of Independent States, the Middle East and North Africa)
FOCUSSED SEGMENTION STRATEGY U.S. RELAUNCH 18 Certified Healthcare Providers Residential Treatment Facilities Criminal Justice System Academic Addiction Programs

CERTIFIED HEALTHCARE PROVIDERS ENGAGE & LEVERAGE THE TOP - TIER 19 Engage the top tier subgroup of 75 - 100 highest Probuphine prescribers Certified Healthcare Providers Residential Treatment Facilities Academic Addiction Programs Criminal Justice System • Enhance reimbursement support and pharmacy coverage • Leverage social media • Partner with advocacy groups to facilitate patient - healthcare provider location matching • Create investigator initiated research programs to generate clinical data and expand awareness • Following success of Probuphine’s relaunch, Titan will pursue commercial partnership to support growth in this segment

RATIONALE & APPROACH 20 RESIDENTIAL TREATMENT FACILITIES Certified Healthcare Providers Residential Treatment Facilities Academic Addiction Programs Criminal Justice System • High rates of post - discharge relapse, including overdose events and related deaths • Growing pressure to include MAT in residential programs • Probuphine is well suited for stable patients returning home Numerous Treatment Facilities in the U.S.

ACADEMIC ADDICITION PROGRAMS INITIAL TARGETING 21 Certified Healthcare Providers Residential Treatment Facilities Academic Addiction Programs Criminal Justice System Thousands of OUD Patients are Treated Annually in Academic Programs Across the U.S. • Initially target selected academic centers, leveraging existing relationships with KOLs • Growth opportunity for Probuphine uptake and clinical research • Train next generation of Probuphine providers • Following initial success, Titan will pursue commercial partnership to support growth in this segment
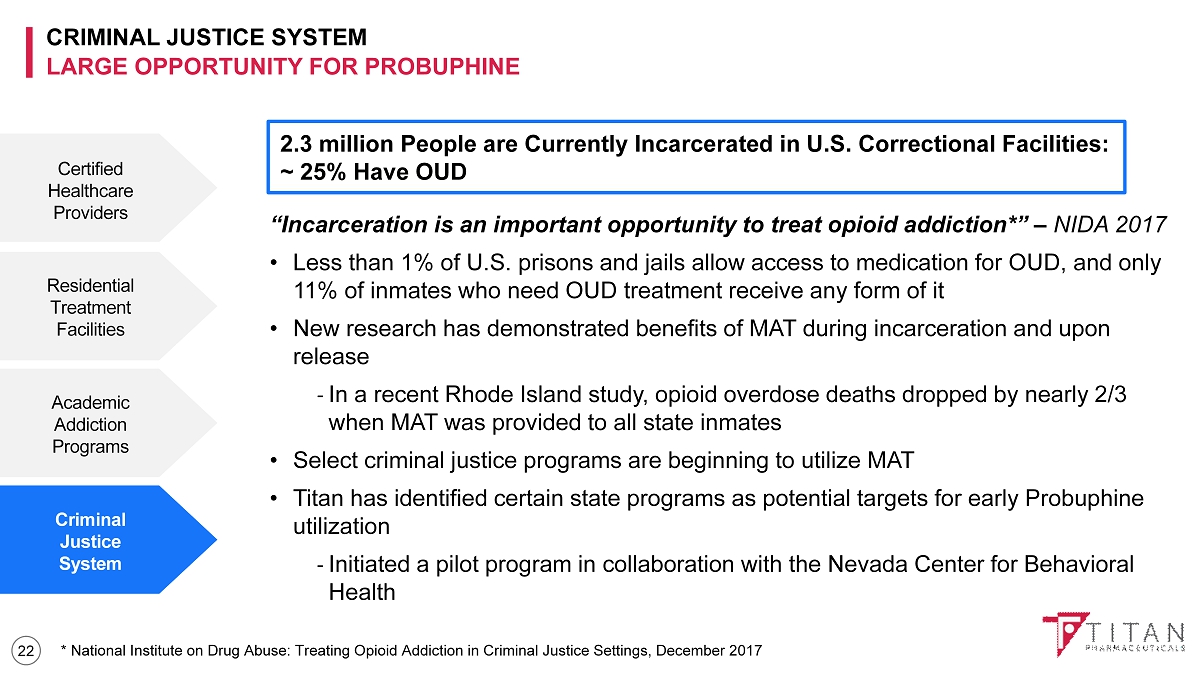
CRIMINAL JUSTICE SYSTEM LARGE OPPORTUNITY FOR PROBUPHINE 22 “Incarceration is an important opportunity to treat opioid addiction*” – NIDA 2017 • Less than 1% of U.S. prisons and jails allow access to medication for OUD, and only 11% of inmates who need OUD treatment receive any form of it • New research has demonstrated benefits of MAT during incarceration and upon release - In a recent Rhode Island study, opioid overdose deaths dropped by nearly 2/3 when MAT was provided to all state inmates • Select criminal justice programs are beginning to utilize MAT • Titan has identified certain state programs as potential targets for early Probuphine utilization - Initiated a pilot program in collaboration with the Nevada Center for Behavioral Health Certified Healthcare Providers Residential Treatment Facilities Academic Addiction Programs Criminal Justice System 2.3 million People are Currently Incarcerated in U.S. Correctional Facilities: ~ 25% Have OUD * National Institute on Drug Abuse: Treating Opioid Addiction in Criminal Justice Settings, December 2017

New FDA guidance on MAT for opioid addiction * • Encourages development of longer - acting formulations • Acknowledges the need for new drugs that don’t end addiction, but help with aspects of it, such as cravings, or overdoses, with the goal remaining complete abstinence Evaluate possible options for broadening the target population • More severe end of the OUD spectrum • Use of Probuphine for long - term taper U.S. REGULATORY STRATEGY ASSESS POTENTIAL FOR LABEL EXPANSION Current indication for Probuphine • Maintenance treatment of OUD in patients who have achieved prolonged clinical stability on low - to - moderate doses of buprenorphine 23 * Press announcement from FDA, April 20, 2018
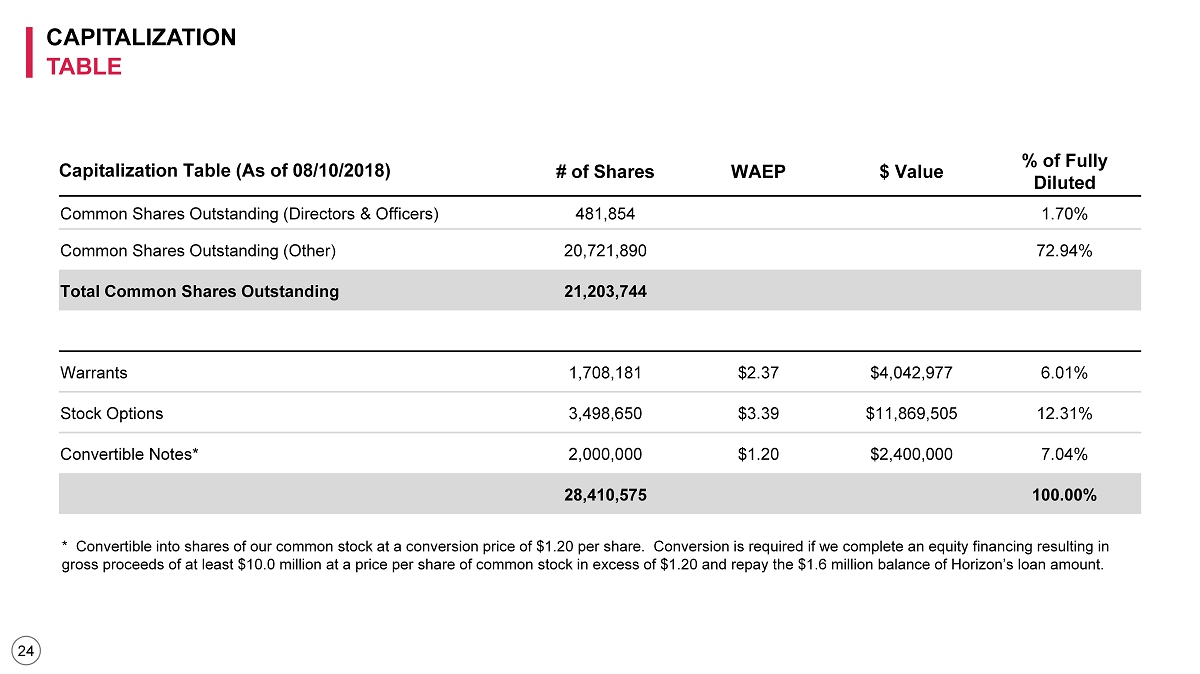
CAPITALIZATION TABLE 24 Capitalization Table (As of 08/10/2018) # of Shares WAEP $ Value % of Fully Diluted Common Shares Outstanding (Directors & Officers) 481,854 1.70% Common Shares Outstanding (Other) 20,721,890 72.94% Total Common Shares Outstanding 21,203,744 Warrants 1,708,181 $2.37 $4,042,977 6.01% Stock Options 3,498,650 $3.39 $11,869,505 12.31% Convertible Notes* 2,000,000 $1.20 $2,400,000 7.04% 28,410,575 100.00% * Convertible into shares of our common stock at a conversion price of $1.20 per share. Conversion is required if we comple te an equity financing resulting in gross proceeds of at least $10.0 million at a price per share of common stock in excess of $1.20 and repay the $1.6 million b ala nce of Horizon’s loan amount.

IN SUMMARY 25 ProNeura: Unique and compelling long - term drug delivery platform Probuphine: Only product on market to provide six - month, continuous, non - fluctuating blood levels of buprenorphine for maintenance treatment of OUD U.S. Commercialization: Probuphine relaunch in early stages Partnerships: Established partnerships for Probuphine in Europe and Canada Development Pipeline : ProNeura - based product candidates for Parkinson’s disease, malaria, hypothyroidism, type 2 diabetes and other conditions

TITAN PHARMACEUTICALS CORPORATE PRESENTATION | SEPTEMBER 2018 © 2018 TITAN PHARMACEUTICALS INC.