
Exhibit 99.1

© 2017 TITAN PHARMACEUTICALS INC. | NASDAQ:TTNP CORPORATE PRESENTATION | SEPTEMBER 2017 TITAN PHARMACEUTICALS

The presentation may contain “forward - looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Reference is made in particular to the description of our plans and objectives for future operations, assumptions underlying such plans and objectives and other forward - looking terminology such as “may,” “expects,” “believes,” “anticipates,” “intends,” “projects,” or similar terms, variations of such terms or the negative of such terms . Forward - looking statements are based on management’s current expectations . Actual results could differ materially from those currently anticipated and such statements involve risks and uncertainties, including, but not limited to, those risks and uncertainties relating to the commercialization of Probuphine , the regulatory approval process, the development, testing, production and marketing of our drug candidates, patent and intellectual property matters and strategic agreements and relationships . ProNeura is a trademark and Probuphine is a registered trademark of Titan Pharmaceuticals, Inc . 2 FORWARD - LOOKING STATEMENTS

3 COMPANY SNAPSHOT ProNeura TM drug delivery platform provides long - term, continuous, non - fluctuating medication levels FDA approved Probuphine ® (buprenorphine) implant: six - month maintenance treatment of opioid addiction • C ommercial partnership with Braeburn Pharmaceuticals for U.S. and Canada; Full U.S. launch start - Q1 2017 • Pursuing approval in EU; Marketing Authorization Application (MAA) submission expected in Q4 2017 Pipeline of additional product candidates from ProNeura platform • Parkinson’s Disease: ropinirole implant in Phase 1/2 clinical study • Hypothyroidism: initial non - clinical testing of T3 implant completed • Collaboration with W RAIR – m alaria prophylaxis • Feasibility evaluations – type 2 diabetes, neuropathic pain
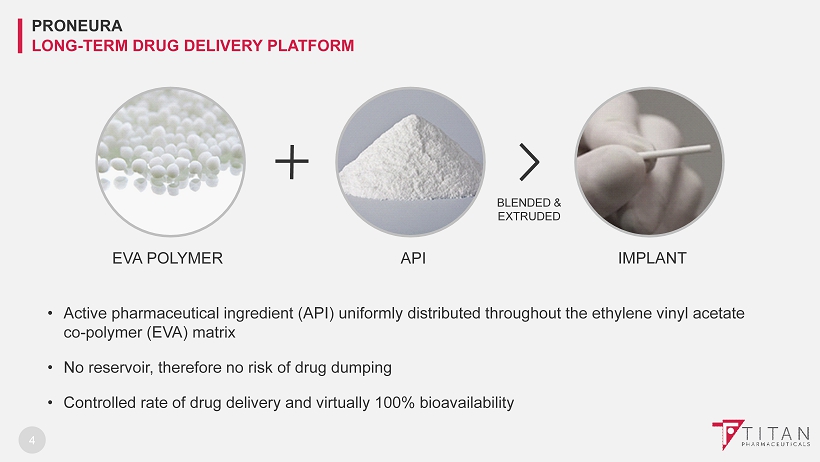
4 • Active pharmaceutical ingredient (API) uniformly distributed throughout the ethylene vinyl acetate co - polymer (EVA) matrix • No reservoir, therefore no risk of drug dumping • Controlled rate of drug delivery and virtually 100% bioavailability EVA POLYMER API IMPLANT BLENDED & EXTRUDED PRONEURA LONG - TERM DRUG DELIVERY PLATFORM
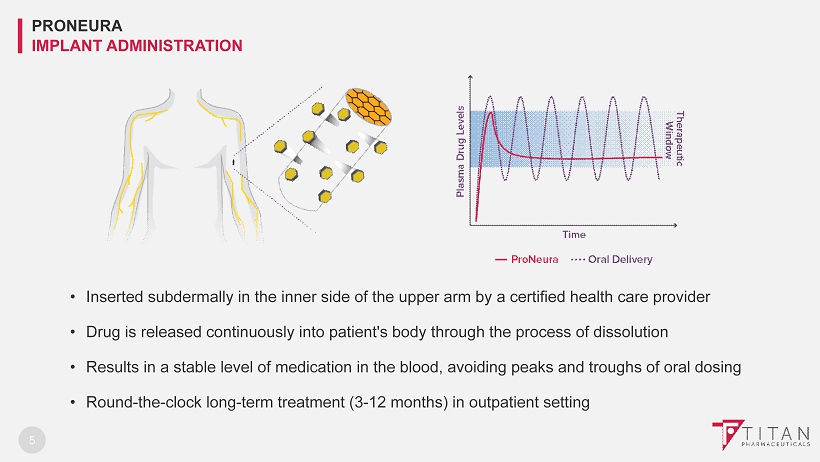
5 • Inserted subdermally in the inner side of the upper arm by a certified health care provider • Drug is released continuously into patient's body through the process of dissolution • Results in a stable level of medication in the blood, avoiding peaks and troughs of oral dosing • Round - the - clock long - term treatment (3 - 12 months) in outpatient setting PRONEURA IMPLANT ADMINISTRATION
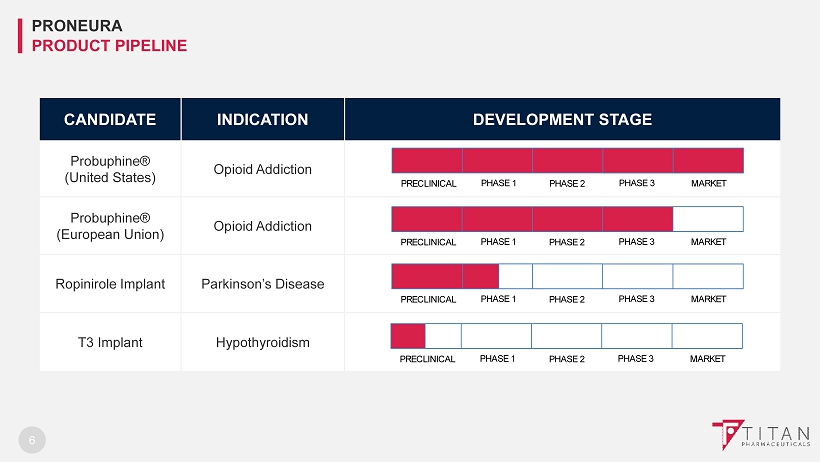
6 CANDIDATE INDICATION DEVELOPMENT STAGE Probuphine ® (United States) Opioid Addiction Probuphine ® (European Union) Opioid Addiction Ropinirole Implant Parkinson’s Disease T3 Implant Hypothyroidism PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET PHASE 2 PRECLINICAL PHASE 1 PHASE 3 MARKET PRONEURA PRODUCT PIPELINE PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET

7 Malaria Prophylaxis: • Collaboration with Walter Reed Army Institute of Research (WRAIR) and Southwest Research Institute (SWRI) • WRAIR interested in developing effective prophylactic treatments for armed forces personnel deployed to malaria endemic regions • Program currently funded by WRAIR and other defense department grants OTHER PRONEURA OPPORTUNITIES Feasibility Evaluations: • Treatment of Type 2 diabetes with currently approved peptides • Treatment of peripheral neuropathic pain with a Kappa opioid receptor agonist • Treatment of opioid addiction with a Mu opioid receptor antagonist
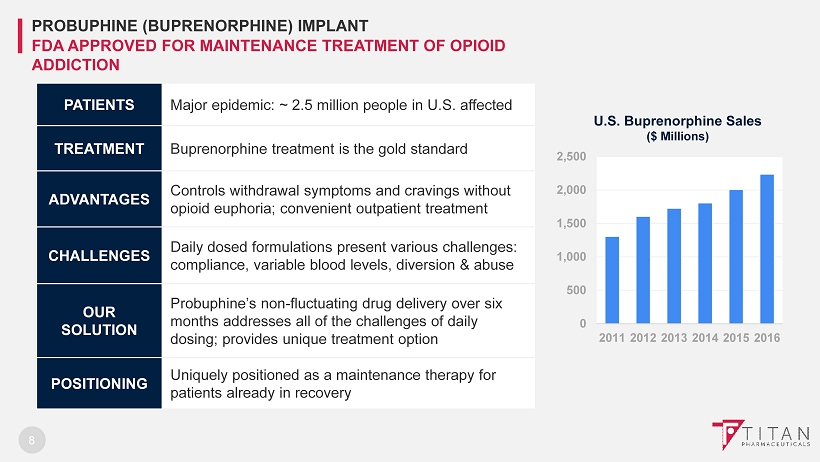
8 PATIENTS Major epidemic: ~ 2.5 million people in U.S. affected TREATMENT Buprenorphine treatment is the gold standard ADVANTAGES Controls withdrawal symptoms and cravings without opioid euphoria; convenient outpatient treatment CHALLENGES Daily dosed formulations present various challenges: compliance, variable blood levels, diversion & abuse OUR SOLUTION Probuphine’s non - fluctuating drug delivery over six months a ddresses all of the challenges of daily dosing ; provides unique treatment option POSITIONING Uniquely positioned as a maintenance therapy for patients already in recovery PROBUPHINE (BUPRENORPHINE) IMPLANT FDA APPROVED FOR MAINTENANCE TREATMENT OF OPIOID ADDICTION 0 500 1,000 1,500 2,000 2,500 2011 2012 2013 2014 2015 2016 U.S. Buprenorphine Sales ($ Millions)

9 Partnership with Braeburn Pharma for U.S. and Canada * signed in December 2012 * Braeburn has sublicensed Canadian rights to Knight Therapeutics PROBUPHINE U.S. COMMERCIALIZATION Milestone payments Upfront: $15.75 million Approval Milestone: $15 million Sales Milestones: up to $165 million Tiered Royalties on net sales Mid - teens to low 20s (%) U.S. Patent term to April 2024

10 PROBUPHINE FULL U.S . COMMERCIAL LAUNCH INITIATED Q1 2017 • Braeburn launched with 60+ field sales force and medical support staff focusing on 80+ key U.S. treatment centers • Status: • 2,500+ health care providers certified under REMS program • 70+ payors indicated coverage intention, including Medicare, Medicaid & VA programs • Braeburn has devoted additional resources to overcome initial challenges • Streamlining paperwork – doctor’s office and 3 rd party payor • Improving order processing • Adding a second specialty pharmacy • Procedure reimbursement • REMS training

11 Recent media coverage includes: • New York Times • Fortune Magazine • CNBC • CBS • Drug Delivery Business News • WPIX - TV (New York, NY) • WMUR Channel 9 (Manchester, NH) • WHDT TV (Stuart, FL) Industry recognition: • Popular Science ‘Best of What’s New’ • Stevie Awards ‘Best New Product ’ PROBUPHINE GAINING AWARENESS

12 Advancing OUS opportunities for regulatory approval and commercial licensing: • Progressing discussions with interested companies in EU and other select regions • March 2017: EMA confirmed eligibility for review and approval under centralized procedure • April 2017: EMA granted a pediatric indication waiver • July 2017: received positive input at pre - MAA meetings from rapporteur (Ireland) and co - rapporteur (UK) country regulatory teams • On track to file MAA with the EMA in Q4 2017 PROBUPHINE OUS COMMERCIALIZATION PLANS
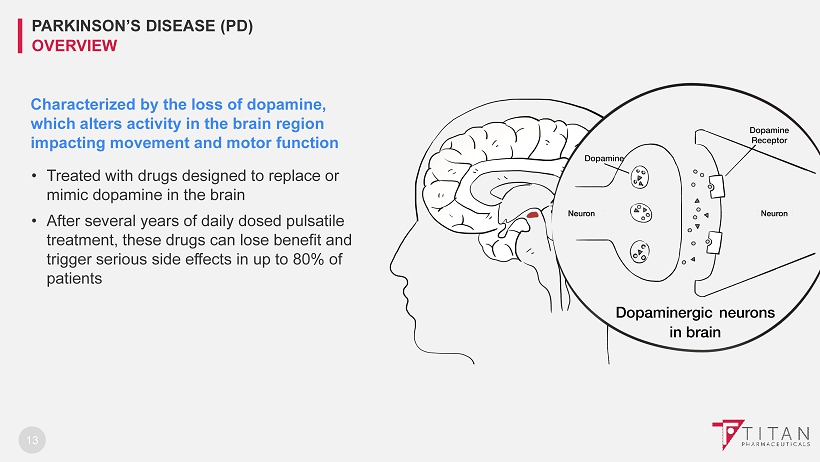
Characterized by the loss of dopamine, which alters activity in the brain region impacting movement and motor function • Treated with drugs designed to replace or mimic dopamine in the brain • After several years of daily dosed pulsatile treatment , these drugs can lose benefit and trigger serious side effects in up to 80% of patients 13 PARKINSON’S DISEASE (PD) OVERVIEW
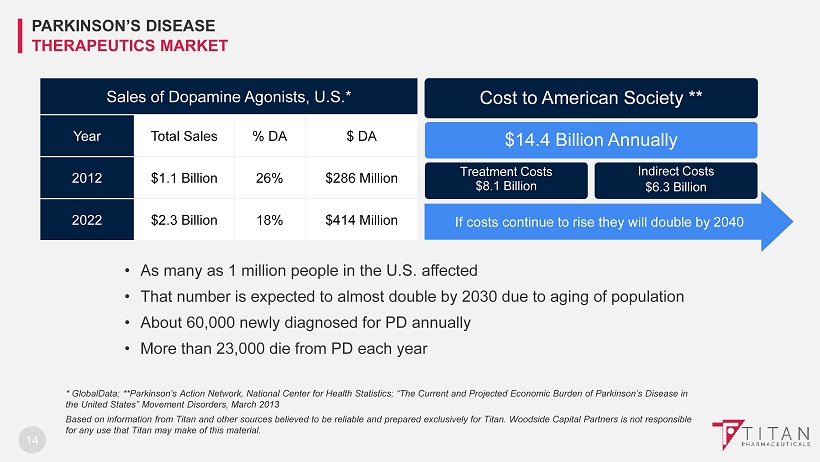
14 * GlobalData; **Parkinson’s Action Network, National Center for Health Statistics; “The Current and Projected Economic Burden of Parkinson’s Disease in the United States” Movement Disorders, March 2013 Based on information from Titan and other sources believed to be reliable and prepared exclusively for Titan. Woodside Capita l P artners is not responsible for any use that Titan may make of this material . Sales of Dopamine Agonists, U.S.* Year Total Sales % DA $ DA 2012 $1.1 Billion 26% $286 Million 2022 $2.3 Billion 18% $414 Million Cost to American Society ** $14.4 Billion Annually Treatment Costs $8.1 Billion Indirect Costs $6.3 Billion If costs continue to rise they will double by 2040 • As many as 1 million people in the U . S . affected • That number is expected to almost double by 2030 due to aging of population • About 60 , 000 newly diagnosed for PD annually • More than 23 , 000 die from PD each year PARKINSON’S DISEASE THERAPEUTICS MARKET
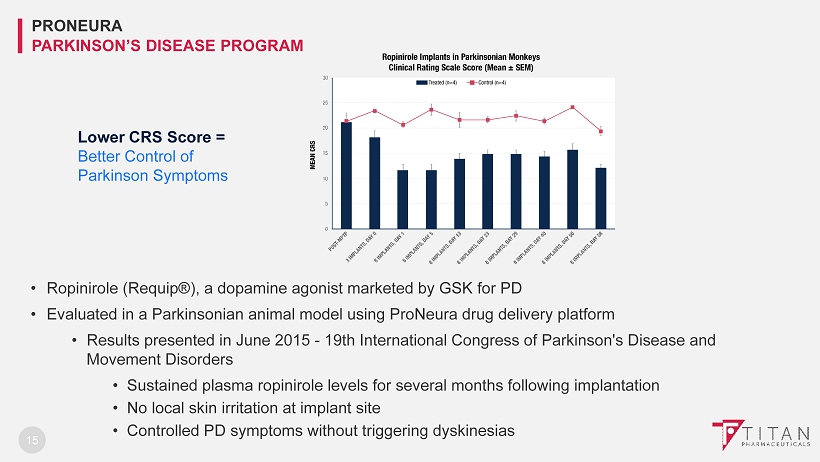
15 • Ropinirole (Requip®), a dopamine agonist marketed by GSK for PD • Evaluated in a Parkinsonian animal model using ProNeura drug delivery platform • Results presented in June 2015 - 19th International Congress of Parkinson's Disease and Movement Disorders • Sustained plasma ropinirole levels for several months following implantation • No local skin irritation at implant site • Controlled PD symptoms without triggering dyskinesias PRONEURA PARKINSON’S DISEASE PROGRAM Lower CRS Score = Better C ontrol of Parkinson Symptoms
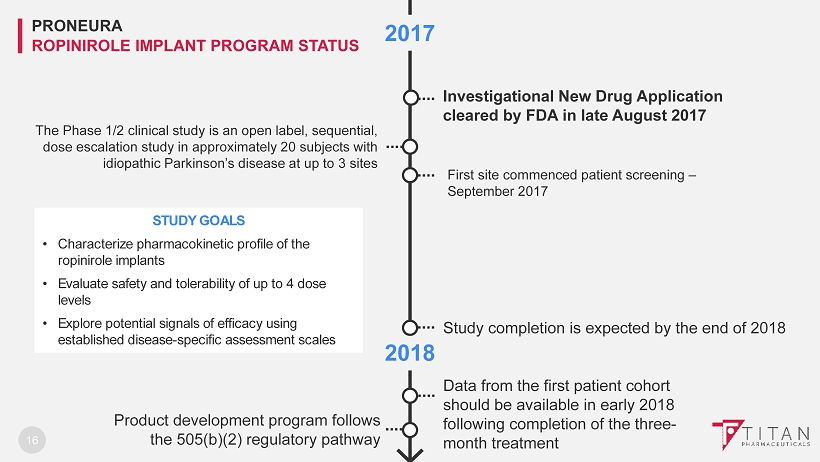
2018 2017 16 Investigational New Drug Application cleared by FDA in late August 2017 PRONEURA ROPINIROLE IMPLANT PROGRAM STATUS Study completion is expected by the end of 2018 Data from the first patient cohort should be available in early 2018 following completion of the three - month treatment Product development program follows the 505(b)(2) regulatory pathway STUDY GOALS • Characterize pharmacokinetic profile of the ropinirole implants • Evaluate safety and tolerability of up to 4 dose levels • Explore potential signals of efficacy using established disease - specific assessment scales The Phase 1/2 clinical study is an open label, sequential, dose escalation study in approximately 20 subjects with idiopathic Parkinson’s disease at up to 3 sites First site commenced patient screening – September 2017

Hypothyroidism: thyroid gland does not make enough hormone to meet the body’s need • Typical treatment: synthetic prohormone thyroxine (T4) given orally once a day • About 15% of patients have difficulty converting T4 to the active triiodothyronine ( T3); in those cases, oral T3 therapy is typically added • Oral T3 treatment is effective, but comes with potential side effects caused by blood level fluctuations 17 OPPORTUNITY IN HYPOTHYROIDISM PRONEURA Animal models have demonstrated ProNeura platform has potential to deliver continuous , non - fluctuating levels of T3 for several months following a single treatment

18 TITAN PHARMACEUTICALS EXECUTIVE MANAGEMENT Marc Rubin, M.D. Executive Chairman & Director • 10 years with Titan • Former Head of Global R&D and member of the Board of Management at Bayer Pharma • Executive R&D and commercial responsibilities at GSK for 13 years • 26 years in the pharmaceutical industry following 7 years at NIH Sunil Bhonsle , M.B.A. President, CEO & Director • 20 years with Titan • 20 years with Bayer Corporation in Biological and Pharmaceutical finance and operations management Kate Beebe, Ph.D. Executive Vice President, Chief Development Officer • 10 years with Titan • 21 years in industry, with senior positions in clinical development and medical affairs at GSK, Merck, and Corcept Therapeutics • 10 years in academic medicine

ProNeura : Unique and compelling long - term drug delivery platform Probuphine : Only product on market to provide six - month, continuous, non - fluctuating blood levels of buprenorphine for maintenance treatment of opioid addiction Development Pipeline : ProNeura - based product candidates for Parkinson’s , Hypothyroidism and other conditions could significantly enhance value 19 TITAN PHARMACEUTICALS SUMMARY Financials: $8.4 million cash as at June 30, 2017, together with $6.8 million from the first tranche of Horizon loan agreement executed in July 2017, sufficient to fund operations into Q1 2019

© 2017 TITAN PHARMACEUTICALS INC. | NASDAQ:TTNP CORPORATE PRESENTATION | SEPTEMBER 2017 THANK YOU. QUESTIONS?