| Sustained anti-pruritic effect in mice with TP- 2021, a kappa opioid agonist peptide, delivered by subdermal ProNeura® Implants B. B. Land1, S. Sreedharan2, R. Patel2, T. Beck3, K. DeVarney2, M. Rubin2, *C. Chavkin1; 1Pharmacol., Univ. of Washington, Seattle, WA; 2Titan Pharmaceuticals, South San Francisco, CA; 3Med. Univ. of South Carolina, Charleston, SC |
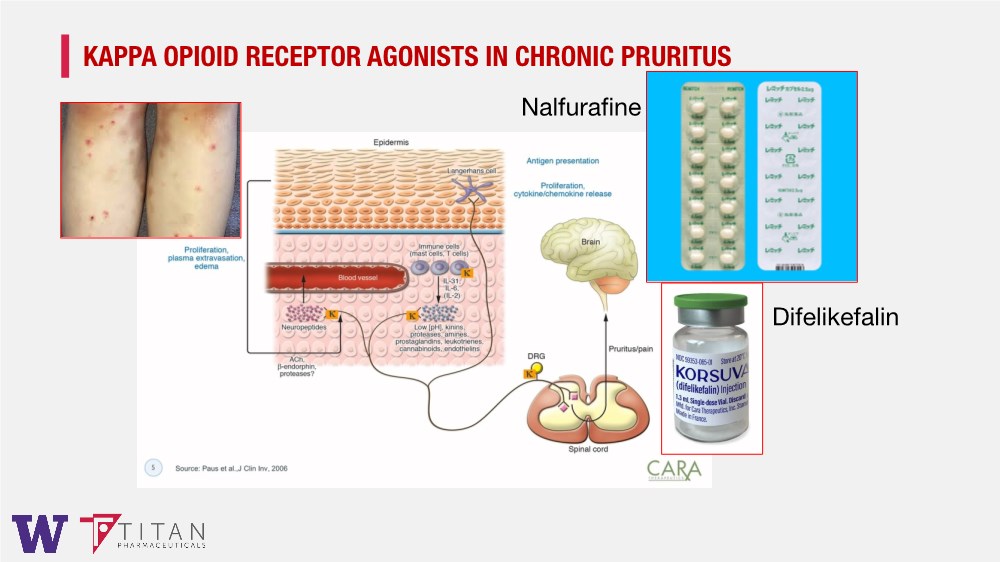
| KAPPA OPIOID RECEPTOR AGONISTS IN CHRONIC PRURITUS Nalfurafine Difelikefalin |
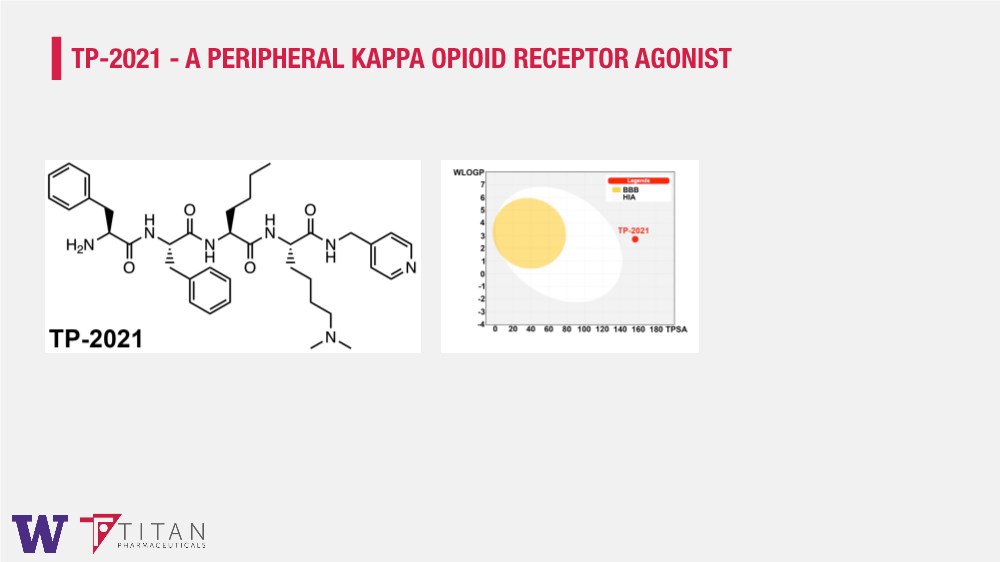
| TP-2021 - A PERIPHERAL KAPPA OPIOID RECEPTOR AGONIST |
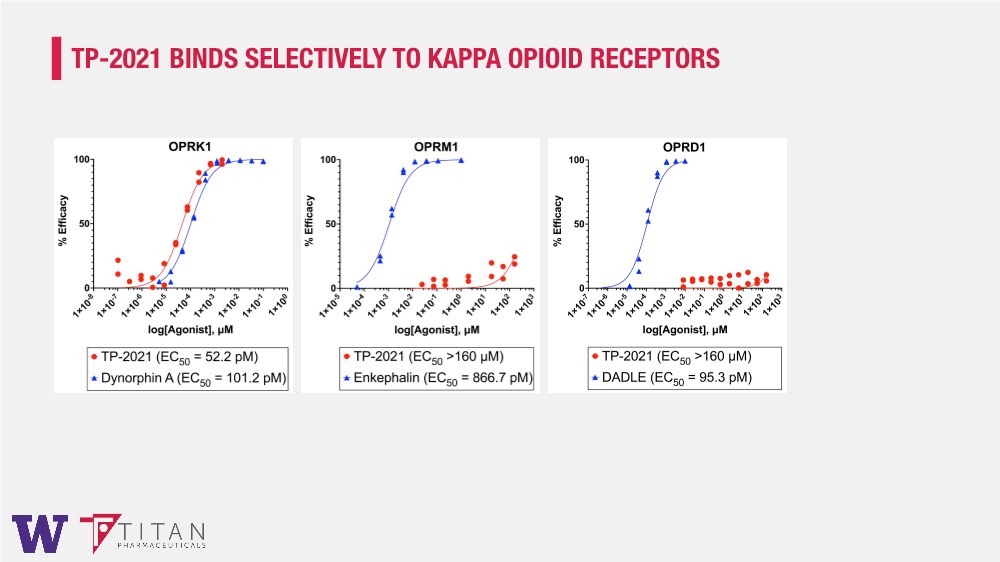
| TP-2021 BINDS SELECTIVELY TO KAPPA OPIOID RECEPTORS |
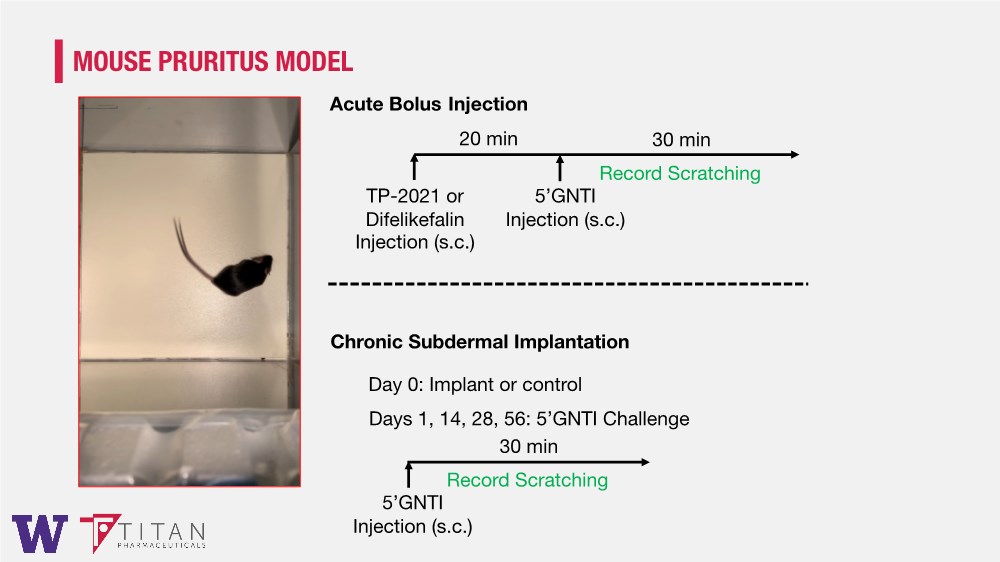
| MOUSE PRURITUS MODEL TP-2021 or Difelikefalin Injection (s.c.) 5’GNTI Injection (s.c.) 30 min 20 min Record Scratching Acute Bolus Injection Chronic Subdermal Implantation 5’GNTI Injection (s.c.) 30 min Record Scratching Day 0: Implant or control Days 1, 14, 28, 56: 5’GNTI Challenge |
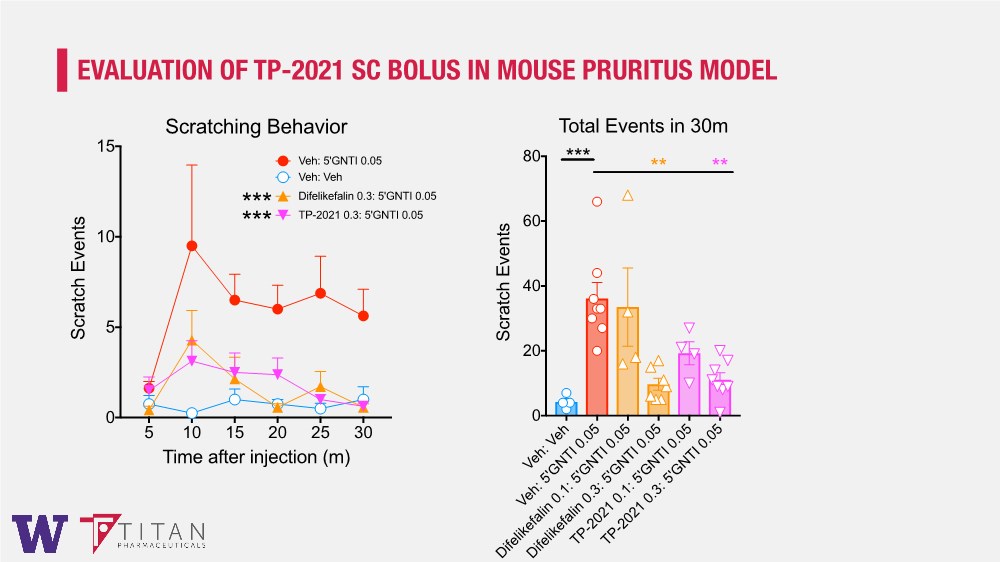
| EVALUATION OF TP-2021 SC BOLUS IN MOUSE PRURITUS MODEL 5 10 15 20 25 30 0 5 10 15 Time after injection (m) Scratch Events Scratching Behavior Veh: 5'GNTI 0.05 Veh: Veh Difelikefalin 0.3: 5'GNTI 0.05 TP-2021 0.3: 5'GNTI 0.05 *** *** Veh: Veh Veh: 5'GNTI 0.05 Difelikefalin 0.1: 5'GNTI 0.05 Difelikefalin 0.3: 5'GNTI 0.05 TP-2021 0.1: 5'GNTI 0.05 TP-2021 0.3: 5'GNTI 0.05 0 20 40 60 80 Scratch Events Total Events in 30m *** ** ** |
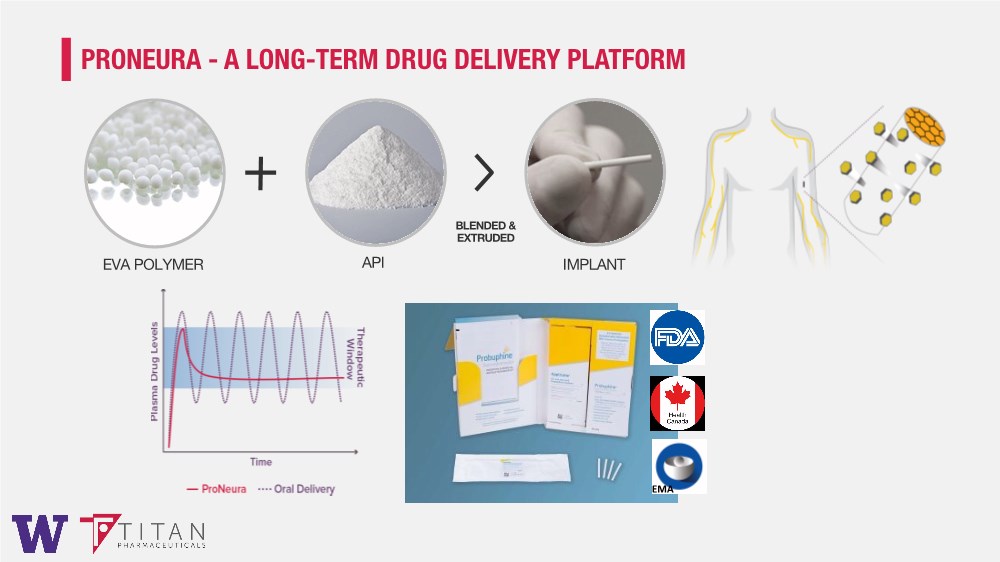
| EVA POLYMER API IMPLANT BLENDED & EXTRUDED PRONEURA - A LONG-TERM DRUG DELIVERY PLATFORM EMA |
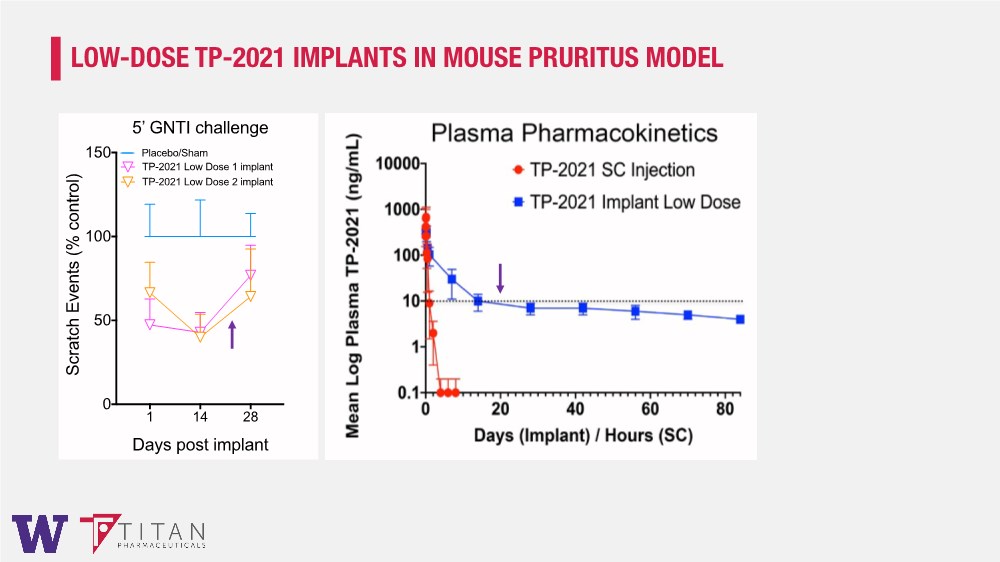
| LOW-DOSE TP-2021 IMPLANTS IN MOUSE PRURITUS MODEL 1 14 28 0 50 100 150 Days post implant Scratch Events (% control) 5’ GNTI challenge Placebo/Sham TP-2021 Low Dose 1 implant TP-2021 Low Dose 2 implant |
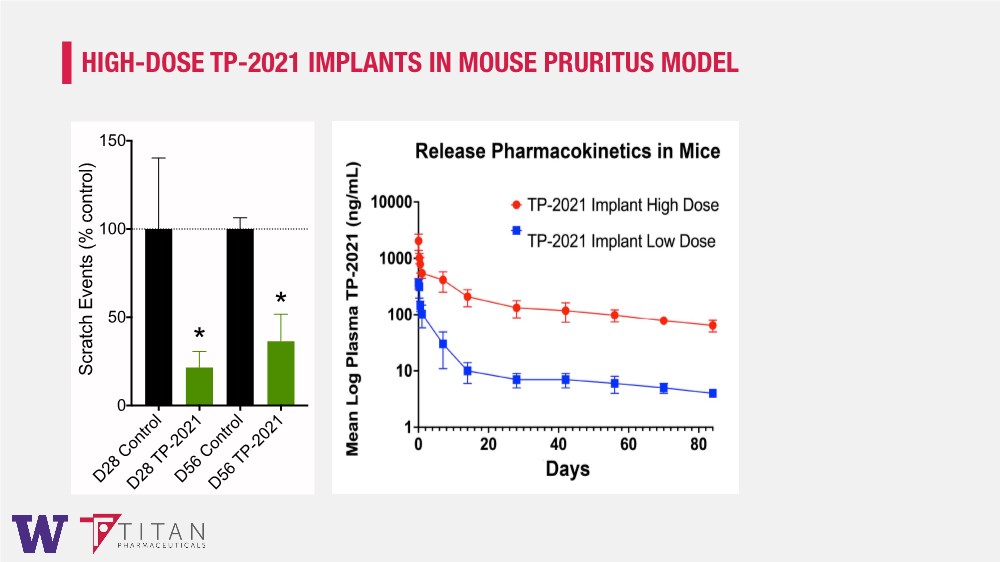
| HIGH-DOSE TP-2021 IMPLANTS IN MOUSE PRURITUS MODEL D28 Control D28 TP-2021 D56 Control D56 TP-2021 0 50 100 150 Scratch Events (% control) * * |
| SUMMARY • TP-2021 is a highly selective and potent kappa opioid receptor agonist • TP-2021 shows similar anti-pruritic efficacy and potency to Difelikefalin when delivered by subcutaneous injection • Low dose subdermal TP-2021-ProNeura implants show anti-pruritic efficacy, which diminishes after 2 weeks • A single high-dose TP-2021-ProNeura implant shows sustained anti-pruritic efficacy, thus far through Day 56, with further efficacy assessments continuing |
| CONCLUSION • TP-2021-ProNeura implants present a viable solution for treatment of chronic pruritus and related conditions for potentially 6 months or longer • A single administration of TP-2021-ProNeura implant could potentially abrogate the need for daily administration, even if a peptide with oral bioavailability is therapeutically achievable |
| CONTRIBUTORS University of Washington Titan Pharmaceuticals Benjamin Land, Ph.D. Charles Chavkin, Ph.D. Sophia Mar Sunil Sreedharan, Ph.D. Raj Patel, Ph.D. Tyler Beck Kate DeVarney, Ph.D. Marc Rubin, M.D. Funding University of Washington Reagents Titan Pharmaceuticals |